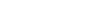ANEWFILL
ANEWFILL - SAFETY
Safety of raw material
Anewfill uses high-quality hyaluronic acid, which is registered by the U.S FDA and CEP certified by the EDQM.
It does not use animal media or enzymes at all during the manufacturing process, reducing the risk of allergic reactions or viruses.
Safety in the manufacturing process
14~15 days of purification Anewfill minimized residual BDDE through a 14-day purification
process and minimized allergic reations by reducing impurities such as endotoxin and protein.
Cross-linked BDDE
Pendant BDDE
Unreacted BDDE
HA
Purification 14~15 DAYS Removal of BDDE residue and impurities using osmotic pressure effect
Comparison of BDDE, Protein load, and Endotoxin detections
| ANEWFILL | A | B | C | |
|---|---|---|---|---|
| BDDE(PPM) | <0.1 | 0.5 | <2 | <0.5 |
| Endotoxin(EU/mL) | <0.5 | 0.5 | 0.2 | 0.2 |
| Protein Load | <0.05 | <0.08 | <0.05 | <0.1 |
| pH | 7.04 | 722 | 7.3 | 7.0 |
ANEWFILL DISINEGRATION TEST
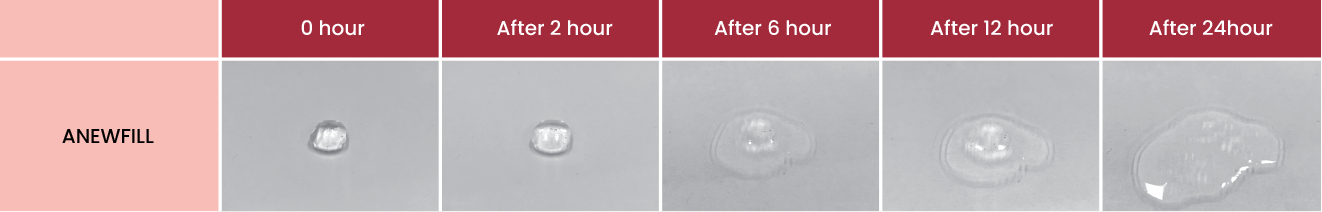
24 hours of observation after hyaluronidase injection into 0.5ml of Anewfill confirmed that it was completely disintegrated.
* If side effects occur after injecting HA fillers, massage lightly after injecting a certain amount of hyaluronidase into the injection area.Do not apply too much pressure.
ANEWFILL - Excellent viscoelasticity
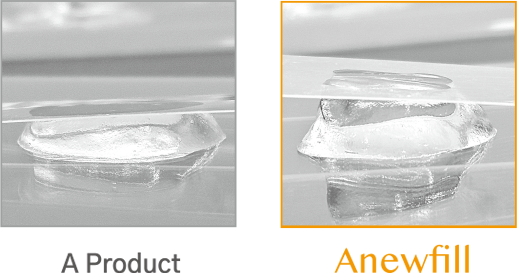
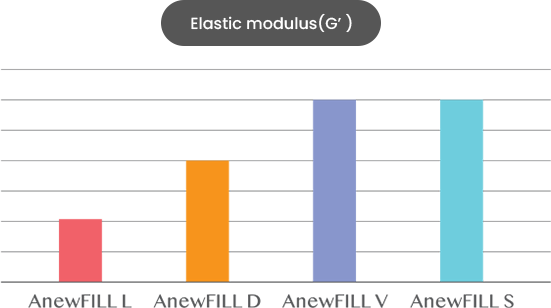
Through much experience, we found the best viscoelasticity for each formulation and applied it to ANEWFILL.
ANEWFILL - Cohesivity
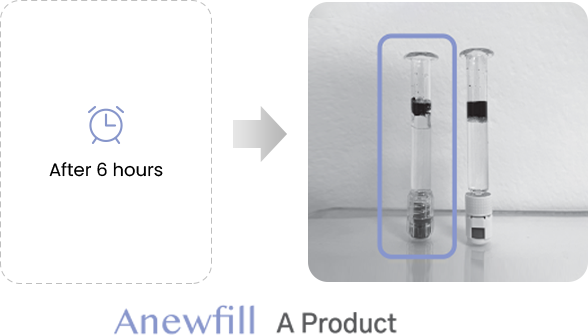
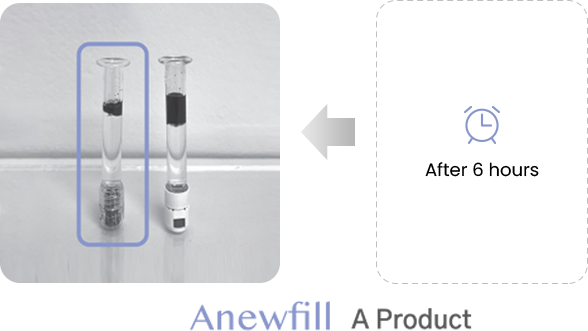
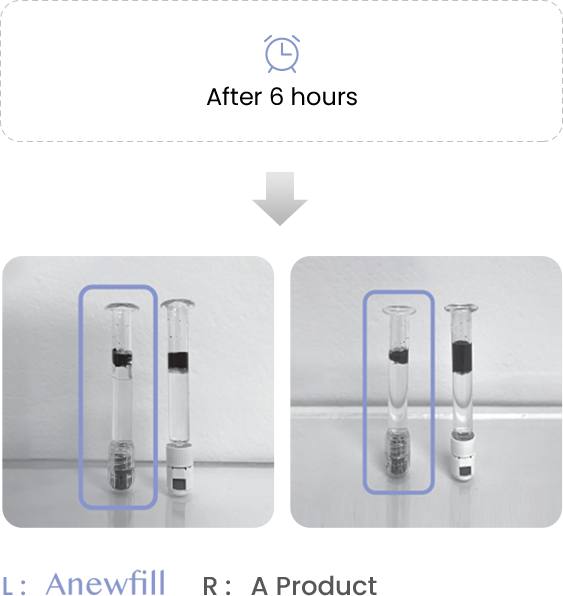
Test the ink diffusion level of Anewfill and other companies’ products.
ANEWFILL - Soft Injection
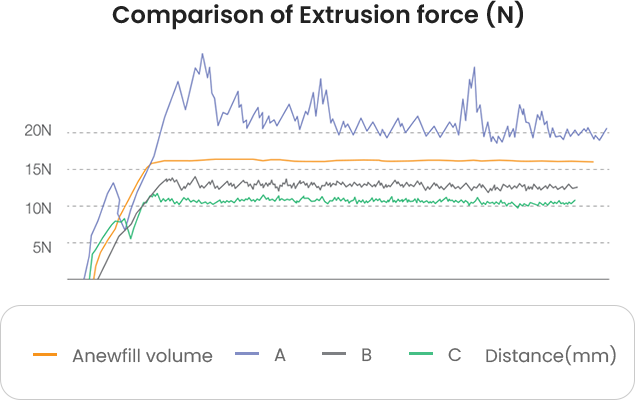
Anewfill can be used easily and smoothly
based on its low and uniform injection force.
based on its low and uniform injection force.
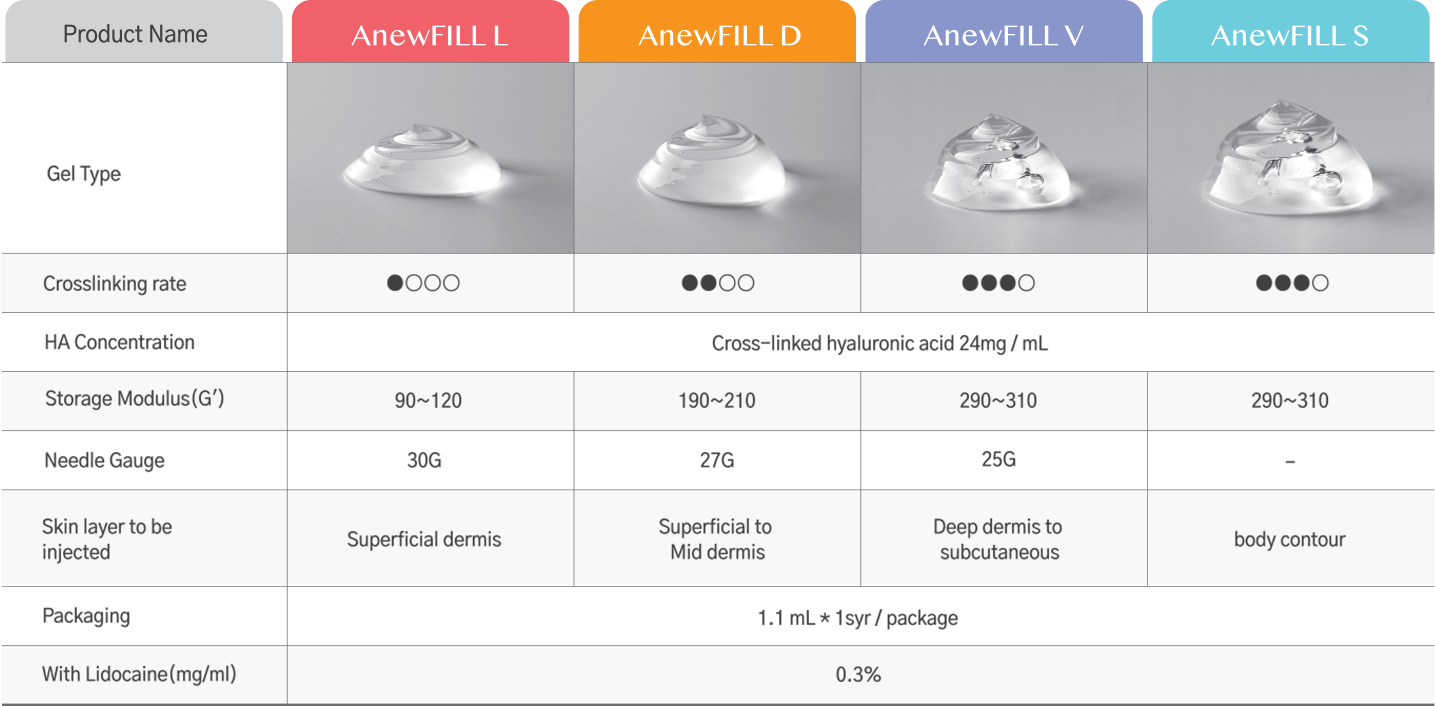
Specification